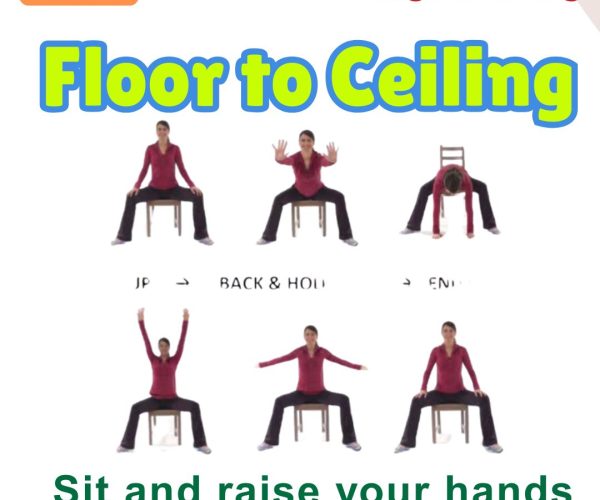
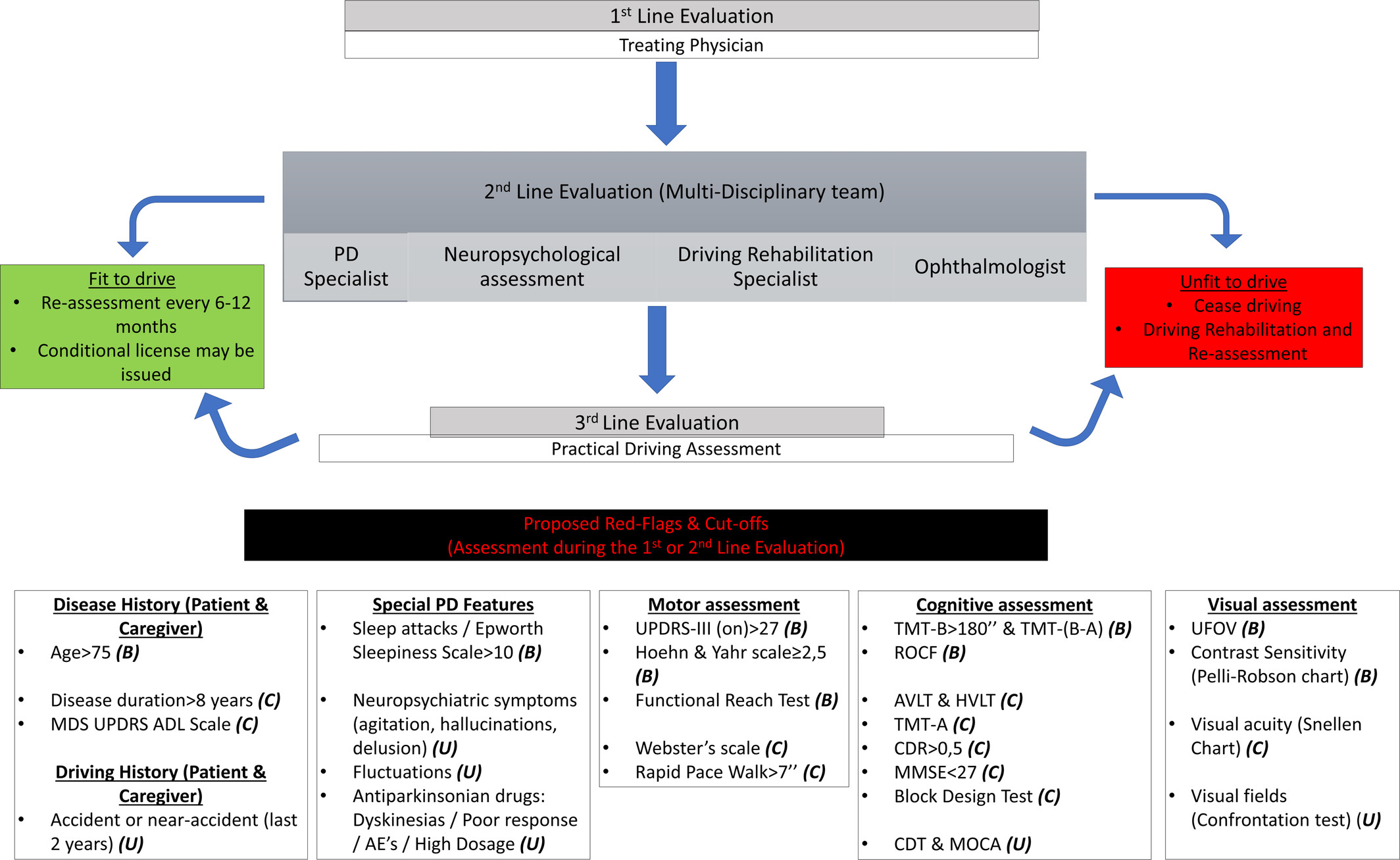
Parkinson’s Disease in 2025: 10 Scientific Breakthroughs That Are Changing the Future of Care
Parkinson’s disease is no longer viewed as a single, static condition—but as a complex, evolving brain disorder influenced by genetics, biology, lifestyle, and time. The year 2025 marked a turning point, with science moving beyond symptom control toward earlier diagnosis, smarter therapies, and the possibility of slowing disease progression. From stem-cell transplants and gene therapies to blood-based biomarkers and non-invasive brain treatments, research in 2025 expanded both the depth and breadth of how Parkinson’s is understood and managed.
This page highlights the Top 10 most important scientific developments in Parkinson’s disease in 2025, explained in a way that connects laboratory discoveries to real-world impact for patients, caregivers, and families—cutting through hype while focusing on what truly matters in care.
Top 10 Breakthroughs in Science for Parkinson's Disease in 2025
10) A next-generation once-daily dopamine agonist (tavapadon) was submitted for FDA review

Original sources: AbbVie NDA submission announcement and PD foundation coverage. AbbVie News Center+1
What it contained (scientific): Regulatory submission seeking approval for tavapadon—positioned as a once-daily oral option for PD (pending review outcomes). Michael J. Fox Foundation+1
Lay implication: The medication toolbox keeps expanding—sometimes the “win” is simpler dosing and smoother day control, not just brand-new mechanisms. Michael J. Fox Foundation+1
How it impacts PD care: If approved, it could offer another way to tailor therapy early and across progression—potentially improving adherence and symptom stability.
9) A new potential neuroprotective candidate (HER-096) reported encouraging early trial signals
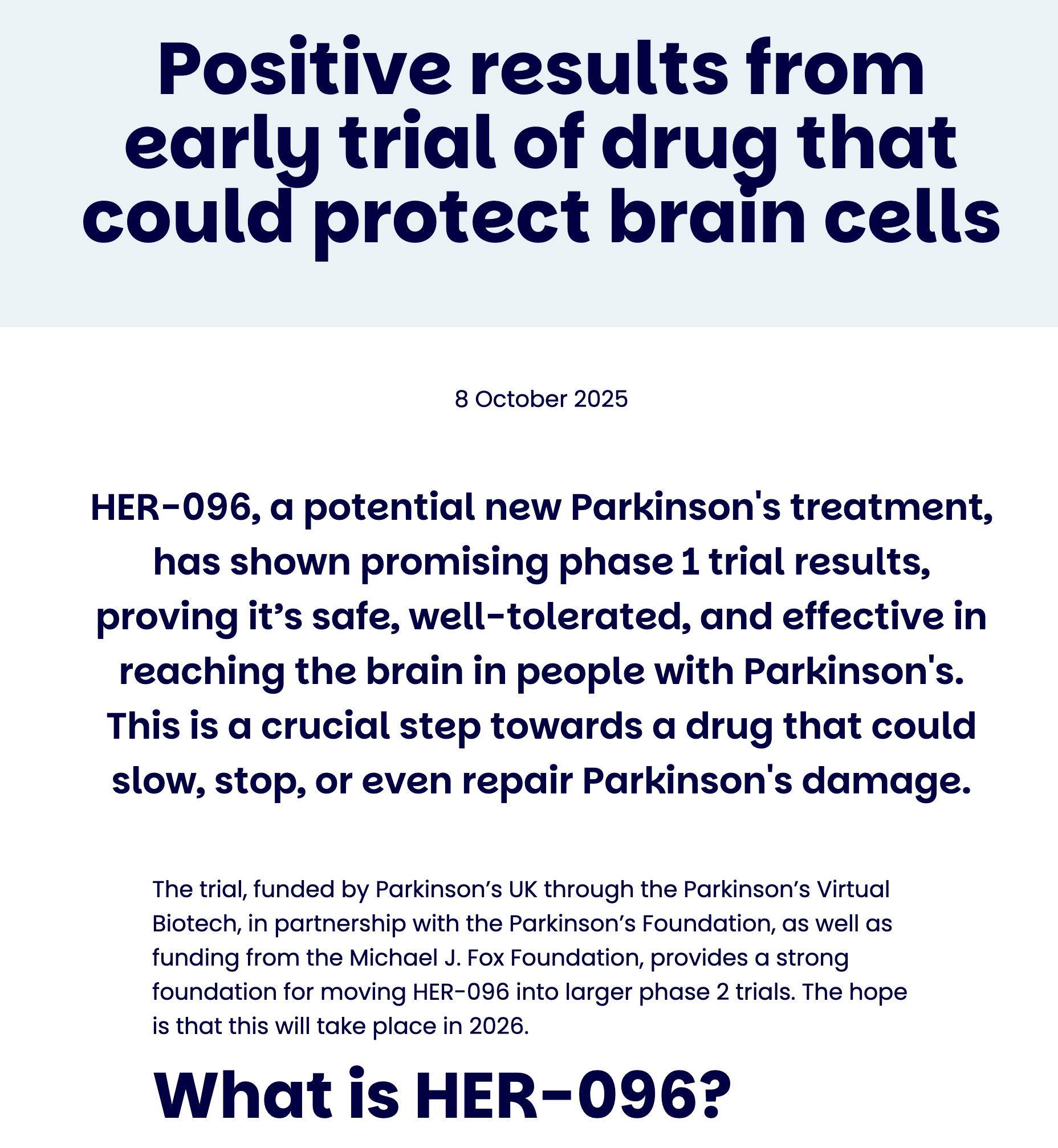
Original source: Parkinson’s UK update (early trial results). Parkinson’s UK
What it contained (scientific): Phase 1 results emphasizing safety/tolerability and evidence the compound reaches the brain—foundational steps for any therapy aiming at neuroprotection/repair. Parkinson’s UK
Lay implication: Before we can prove a drug slows PD, we first prove it’s safe and actually gets to where it needs to act. Parkinson’s UK
How it impacts PD care: Not a clinic treatment yet, but it expands the pipeline of “protect the brain” approaches beyond dopamine replacement.
8) A gene-therapy program (AB-1005) published Phase 1b results and moved the field forward

Original source: AskBio publication announcement of Phase 1b AB-1005 gene therapy results in Movement Disorders (company summary). AskBio
What it contained (scientific): Early clinical data focusing on safety/tolerability and feasibility of gene therapy delivery; positioned as a potential approach to slow progression (still investigational). AskBio
Lay implication: Gene therapy for PD is no longer “science fiction”—but it’s still in the careful, step-by-step proof stage. AskBio
How it impacts PD care: Mostly through trial availability and accelerating know-how about dosing, delivery, and long-term monitoring.
7) Another large biomarker push suggested panels (blood/urine markers) linked to PD risk years before diagnosis

Original science publication: Biomarker screening work in npj Parkinson’s Disease (2025). nature.com
What it contained (scientific): Screening many routine biomarkers, narrowing to a subset associated with PD, including several highlighted as strongly associated in that dataset. nature.com
Lay implication: PD risk may eventually be estimated using combinations of routine lab patterns—like “risk fingerprints,” not a single magic number. nature.com
How it impacts PD care: Supports the idea that future PD care may include risk staging (like cardiology risk scores), particularly for research enrollment and prevention trials.
6) A blood-based RNA signal for very early PD detection was reported (pre-motor stage potential)
Original science publication / coverage: RNA/tRNA-fragment blood test work reported in Nature Aging (as covered by major PD organizations). American Parkinson Disease Association+1
What it contained (scientific): A method to detect PD-associated RNA fragment patterns in blood—aiming to identify PD before classic motor symptoms appear. American Parkinson Disease Association
Lay implication: This is part of the race toward a “blood test for Parkinson’s,” especially for early detection. American Parkinson Disease Association
How it impacts PD care: Not ready for routine clinic use, but it strengthens future pathways for:
-
screening high-risk people, and
-
getting patients into disease-modifying trials earlier.
5) Roche decided to take an anti-alpha-synuclein antibody (prasinezumab) forward into Phase 3

Original source: Roche announcement about advancing prasinezumab into Phase III based on trends and biomarker signals. Roche
What it contained (scientific): Reported signals (not a “cure claim”) suggesting reduced motor progression trends over 2 years in a study population and biomarker evidence consistent with target engagement/biology impact. Roche
Lay implication: A serious attempt at disease-modifying therapy (slowing progression), not just symptom relief, is being pushed into the definitive trial stage. Roche
How it impacts PD care: While not yet available clinically, it keeps the field focused on earlier diagnosis + earlier intervention strategies if/when such therapies prove effective.
4) A new continuous apomorphine infusion option got FDA approval for advanced PD “OFF” episodes

Original source: FDA approval announcement for ONAPGO (SPN-830) subcutaneous apomorphine infusion. Supernus+2Reuters+2
What it contained (scientific): Continuous subcutaneous delivery of apomorphine (dopamine agonist) aimed at reducing motor fluctuations; late-stage data showed meaningful reduction in daily OFF time vs placebo. Reuters+1
Lay implication: Instead of repeated rescue doses, medication can be delivered more steadily—helping reduce “wearing off.” Reuters
How it impacts PD care: More personalization for advanced PD—especially when oral absorption is erratic or OFF time dominates the day.
3) FDA cleared staged bilateral focused ultrasound for advanced PD symptoms (non-incision brain procedure)

Original announcement(s): FDA approval/clearance communications around staged bilateral focused ultrasound in advanced PD (foundation and manufacturer communications; clinical center explainer). Michael J. Fox Foundation+3FUS Foundation+3Insightec+3
What it contained (scientific): Expansion from one-sided to two-sided (staged) treatment using MRI-guided focused ultrasound to lesion a target pathway/region to reduce severe symptoms when medications fail. Insightec+1
Lay implication: A “no cut, no implant” option expanded—potentially helping people whose symptoms are disabling on both sides of the body. NewYork-Presbyterian+1
How it impacts PD care: Adds another advanced-therapy choice alongside DBS and infusion therapies—important for those who cannot (or do not want to) undergo implanted-device surgery.
2) A major stem-cell program moved into Phase 3 testing (closer to real-world approval pathways)

Original report: Bayer/BlueRock moved its PD cell therapy into Phase III trials (news report). Reuters
What it contained (scientific): Progression from earlier safety/tolerability and “cells functioning as intended” signals into the pivotal trial stage aimed at regulatory approval. Reuters
Lay implication: This is the step where treatments start being tested at the scale and rigor needed to become widely available. Reuters
How it impacts PD care: You may see more centers discussing trial eligibility, referral pathways, and long-term follow-up planning for restorative therapies.
1) Stem-cell dopamine neuron transplants showed encouraging early human results
Original science publication(s): Two clinical trials published in Nature (one using iPSC-derived dopamine progenitors; one using hES-derived dopamine neuron progenitors). nature.com+1
What it contained (scientific): Early-phase trials evaluating safety, cell survival, dopamine function (imaging evidence), and exploratory clinical changes after transplanting dopamine-producing precursor cells into the brain’s target regions. nature.com+2nature.com+2
Lay implication: “Replacing the missing dopamine cells” is moving from theory toward real-world feasibility—without tumors or major safety signals in these small studies. Parkinson’s Foundation+2nature.com+2
How it impacts PD care: Not a routine treatment yet, but it meaningfully strengthens the case that cell replacement could become a future option for selected patients—especially when symptoms are no longer well controlled by tablets alone.
To Conclude
Taken together, the breakthroughs of 2025 tell an important story: Parkinson’s care is moving from a reactive, symptom-based approach toward a future that is predictive, personalized, and potentially disease-modifying. While many of these advances are still in clinical trials and not yet part of routine treatment, they collectively reshape how Parkinson’s is diagnosed, monitored, and treated across its full journey. For patients and families, this means more informed choices, earlier intervention opportunities, and a growing pipeline of therapies aimed not just at living with Parkinson’s—but living better with it. Science does not change care overnight, but 2025 has clearly laid stronger foundations for the years ahead.
Acknowledgment
The identification of scientific publications and developments summarized on this page was assisted by AI-based search and aggregation tools to scan the 2025 Parkinson’s disease literature landscape. All shortlisted studies were manually reviewed, interpreted, and contextually evaluated by the author, and the final selection of the “Top 10” breakthroughs was made solely by the author based on scientific rigor, clinical relevance, and potential impact on patient care.





 A major turning point came in 1948, when John Nathaniel Cumings demonstrated that patients with Wilson’s disease had massive copper accumulation in both the liver and brain. This firmly established the illness as a disorder of copper metabolism.Cumings proposed a daring idea: if copper was the toxin, perhaps it could be removed. He turned to a wartime antidote – British Anti-Lewisite (BAL), a chelating agent developed during World War II as a treatment for arsenical gas exposure.
A major turning point came in 1948, when John Nathaniel Cumings demonstrated that patients with Wilson’s disease had massive copper accumulation in both the liver and brain. This firmly established the illness as a disorder of copper metabolism.Cumings proposed a daring idea: if copper was the toxin, perhaps it could be removed. He turned to a wartime antidote – British Anti-Lewisite (BAL), a chelating agent developed during World War II as a treatment for arsenical gas exposure.


 Wilson’s original description did much more than define a single rare disease. By showing that movement disorders could arise from specific basal ganglia pathology, he helped reshape thinking about Parkinson’s disease, Huntington’s disease, dystonias, and many other conditions.Over the 20th century, this conceptual framework evolved into a full-fledged subspecialty: Movement Disorders Neurology. Treatments such as levodopa therapy, botulinum toxin for dystonia, and deep brain stimulation for Parkinson’s disease all rest on the foundation that Wilson’s meticulous clinico-pathological work helped to build.
Wilson’s original description did much more than define a single rare disease. By showing that movement disorders could arise from specific basal ganglia pathology, he helped reshape thinking about Parkinson’s disease, Huntington’s disease, dystonias, and many other conditions.Over the 20th century, this conceptual framework evolved into a full-fledged subspecialty: Movement Disorders Neurology. Treatments such as levodopa therapy, botulinum toxin for dystonia, and deep brain stimulation for Parkinson’s disease all rest on the foundation that Wilson’s meticulous clinico-pathological work helped to build.
 Warning Signs That Should Raise Suspicion
Warning Signs That Should Raise Suspicion